The work introduces a catalyst-guided cascade oxidation mechanism that substantially reduces energy input, opening new possibilities for integrating solar hydrogen production with sustainable biomass upgrading.
The researchers began by comparing earth-abundant metal oxyhydroxides and identified CoOOH as a promising starting point for glucose oxidation. They then systematically introduced various dopants and discovered that adding just 5 mol% copper transformed CoOOH into a far more selective and efficient electrocatalyst. With this modification, the yield of format increased from 50% to 80%, and the onset potential for glucose oxidation dropped by about 400 mV, enabling highly energy-efficient co-electrolysis in alkaline conditions.
A suite of advanced characterization techniques, including X-ray photoelectron spectroscopy, Raman spectroscopy, electron microscopy, and in situ impedance analysis, revealed how copper reshapes the electronic landscape of the catalyst surface.
Copper stabilizes reactive Co³⁺ sites while suppressing overly aggressive Co⁴⁺ species that typically lead to non-selective bond cleavage. Complementary DFT calculations showed that Cu doping disfavors side-on adsorption of glucose and suppresses β-cleavage pathways that form by-products. Instead, it promotes end-on binding at the aldehyde group, enabling a stepwise α-C–C cleavage sequence that releases format from every carbon atom.
When paired with an earth-abundant Ni₄Mo cathode, the system produced pure hydrogen in a membrane-free cell with nearly 100% Faradaic efficiency. Under concentrated sunlight, the device achieved a hydrogen generation rate of 519.5 ± 0.4 μmol h⁻¹ cm⁻², maintaining stable performance across 24 hours of operation.
One of the study's senior researchers noted that the findings illustrate how the catalyst design can reshape both the efficiency and economics of solar hydrogen production. By orchestrating glucose oxidation through a highly selective α-cleavage pathway, the catalyst not only reduces the electrical energy required but simultaneously upgrades biomass into a valuable chemical feedstock. This dual-function system, the expert emphasized, represents a pivotal shift toward more integrated and cost-effective renewable hydrogen technologies, demonstrating that sustainable chemistry and clean energy generation can be mutually reinforcing.
This co-electrolysis strategy offers a scalable and economically competitive route to green hydrogen by pairing energy-efficient operation with the sale of format as a co-product. Economic modeling suggests that this approach could lower the levelized cost of hydrogen to $1.54 per kilogram, rivaling or undercutting hydrogen produced from fossil fuels. The membrane-free design also simplifies the system architecture and reduces capital costs, making industrial deployment more feasible. Importantly, the catalyst performs equally well on hydrolysates derived from agricultural waste, highlighting its compatibility with real-world biomass resources and its potential to support distributed hydrogen production in future circular bioeconomy systems.
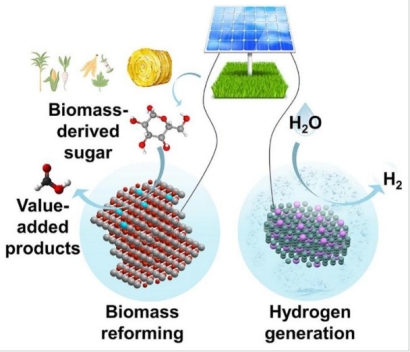
CAPTION
This schematic illustrates the solar-powered co-electrolysis strategy in which biomass-derived sugars undergo selective oxidation on a copper-doped cobalt oxyhydroxide catalyst to yield value-added products such as format. Simultaneously, water is reduced at the cathode to generate high-purity hydrogen in a membrane-free reactor. The integrated process upgrades renewable biomass while reducing the energy demand of hydrogen production, offering a cost-effective pathway for green fuel generation.

